Introduction
Thyrotoxicosis is the clinical manifestation of excess thyroid hormone action at the tissue level due to inappropriately high circulating thyroid hormone concentrations.
Hyperthyroidism is a subset of thyrotoxicosis, referring specifically to excess thyroid hormone synthesis and secretion by the thyroid gland.
The most common cause of thyrotoxicosis is Grave’s disease, an autoimmune condition which results in excess endogenous production of thyroid hormones.1
Aetiology
Anatomy
The thyroid gland is located within the neck at the level of the C5-T1 vertebrae.
It consists of a central isthmus and two lobes, to the left and the right. The gland sits anterior to the trachea with the four parathyroid glands, which sit on the posterior surface of the thyroid gland.2
Physiology
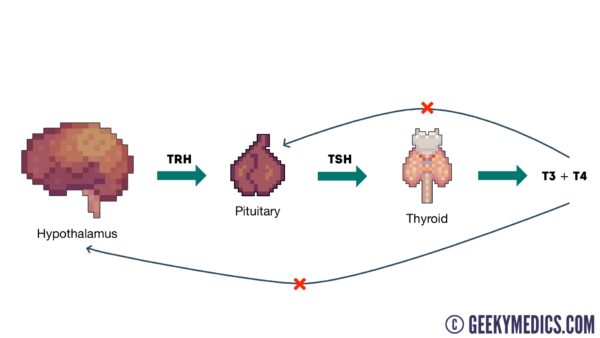
Thyroid hormones are produced from follicular cells of the thyroid gland. Production of these hormones is regulated via the hypothalamic-pituitary-thyroid (HPT) axis (Figure 1).
Thyrotropin-releasing hormone (TRH) is released from the paraventricular nucleus of the hypothalamus. TRH binds to pituitary receptors and causes the production of thyroid-stimulating hormone (TSH) from pituitary cells known as thyrotrophs.
TSH subsequently binds to receptors on the thyroid gland and causes the production of thyroid hormones from the thyroid follicular cells. These hormones are triiodothyronine (T3) and thyroxine (T4).
The HPT axis is regulated via negative feedback, at the level of the hypothalamus and the pituitary.
Excess concentrations of thyroid hormones feedback to both the hypothalamus and the pituitary to cause a reduction in both TRH and TSH production. This decreases the rate of thyroid hormone production via the HPT axis. This physiological regulation of the HPT axis is lost in thyrotoxicosis.3
T4 is the main hormone produced from the thyroid gland, alongside smaller amounts of T3. The majority of T4 is then converted to T3 when it reaches the target tissue (this is the most active form of the hormone).4
Thyroid hormones have a wide range of functions including influencing the basal metabolic rate (the rate of cellular oxidative phosphorylation).
Increased thyroid hormone concentration (seen in thyrotoxicosis) increases the basal metabolic rate. Thyroid hormones also have a key role in physiological growth, including fetal growth (thyroid hormones mediate the growth of neuronal and dendritic cells within the fetal central nervous system) and paediatric growth (thyroid hormones stimulate the expression of the pituitary growth hormone).5

Causes of thyrotoxicosis
Grave’s disease
Grave’s disease is the most common cause of thyrotoxicosis and hyperthyroidism. This is an autoimmune condition mediated via anti-TSH-Receptor (anti-TSHR) autoantibodies.
These autoantibodies bind to pituitary TSH-receptors and stimulate increased production of TSH from the pituitary gland, independent of TRH concentrations.
The increased serum TSH causes increased production of downstream T3 and T4 from the thyroid gland, resulting in thyrotoxicosis.
The prevalence of Grave’s disease in the UK is 0.5%, typically presenting in patients between the ages of 40 and 60, and is much more common in female patients. Grave’s disease is also strongly associated with other auto-immune conditions including type 1 diabetes mellitus, Addison’s disease and vitiligo.4
Toxic multinodular goitre
Toxic multinodular goitre (TMG) is caused by the development of physiologically active nodules on the thyroid gland, which are capable of secreting thyroid hormones. These nodules are not responsive to circulating TSH concentrations and so, eventually cause thyrotoxicosis.
TMG is the second commonest cause of hyperthyroidism and most commonly affects older patients. Thyrotoxicosis due to a toxic multinodular goitre typically has a more insidious onset, in comparison to Grave’s disease.6
Iodine excess
Excess serum iodine concentrations can also cause thyrotoxicosis. This is because thyroid hormone production is dependent upon iodine, so increased serum iodine concentrations allow increased production of the hormones.
Iodine excess can occur following the use of contrast media for imaging modalities or via the contamination of food.1
Iatrogenic
Iatrogenic causes of thyrotoxicosis include the drugs amiodarone and levothyroxine.
Amiodarone contains increases iodine levels and so increases follicular thyroid hormone production. Levothyroxine is used in the management of hypothyroidism to increase thyroid hormone production where there is a deficit. Overuse of this drug can cause an inappropriately increased serum hormone concentration.1
Viral infection
Viral infections can predispose to a phenomenon known as ‘subacute De Quervain’s thyroiditis’. This can cause a transient rise in thyroid hormone production due to inflammation of the thyroid gland and subsequent excessive excretion of thyroid hormones into the circulation. subacute De Quervain’s thyroiditis presents with a painful lump in the neck, most commonly between the ages of 20-50.4
Patients may subsequently develop hypothyroidism if the thyroid gland tissue is damaged by the inflammation.
Postpartum thyroiditis
Postpartum thyroiditis presents with a transient acute phase of thyrotoxicosis, followed by a period of hypothyroidism. This can occur 2 – 6 months following birth or miscarriage.7
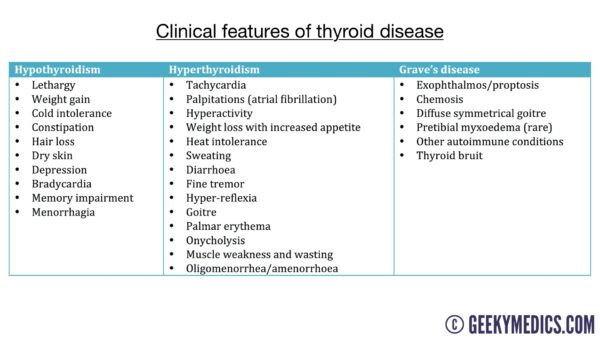
Clinical features
Thyrotoxicosis presents with a wide range of clinical features due to the overall increased basal metabolic rate.
History
Typical symptoms of thyrotoxicosis include:
- Recent unintended weight loss
- Increased appetite
- Diarrhoea
- Heat intolerance (patients may appear underdressed for the weather)
- Over-activity and restlessness
- Tremor
- Palpitations
- Irritability
- Muscle weakness
- Loss of libido
- Oligomenorrhoea
Other important areas to cover in the history include:
- Past medical history: autoimmune conditions (Grave’s disease) or recent viral infection (De Quervain’s thyroiditis)
- Family history: family history of Grave’s disease
- Medication history: use of amiodarone, levothyroxine, or recent use of contrast media
- Obstetric history: recent pregnancy or miscarriage
Clinical examination
A thyroid status examination should be performed in all patients presenting with symptoms suggestive of thyrotoxicosis.
Clinical features of thyrotoxicosis may include:1
- Thin and brittle hair
- Loss of the outer third of the eyebrow
- Warm and moist skin
- Irregular or fast heart rate
- Fine tremor
- Brisk reflexes
- Palmar erythema
- Lid lag and lid retraction
- Goitre (enlargement of the neck due to an enlarged thyroid gland)
Grave’s disease
There are some clinical features of thyrotoxicosis which are specific to Grave’s disease, due to the presence of the anti-TSHR autoantibodies.
Clinical features of Grave’s disease may include:4,8
- Thyroid eye disease (Grave’s ophthalmopathy): conjunctival injection, aching at the back of the eye, diplopia, gradual proptosis (exophthalmos), lid retraction, lid lag, chemosis (oedema of the eye)
- Thyroid acropachy: clubbing or swelling of the digits
- Pretibial myxoedema: oedema of the pretibial portion of the leg (just above the lateral malleolus)



Investigations
Thyroid function tests
The key laboratory investigation for the diagnosis of any thyroid condition is thyroid function tests (TFTs) which include serum levels of TSH, T3 and T4.
In most cases of thyrotoxicosis, the pattern of TFTs will reflect an increased production of thyroid hormones from the thyroid gland and a decreased production of pituitary TSH.
This is because the HPT axis will attempt to correct the excess thyroid hormone production via negative feedback:
- Increased T3 and T4
- Decreased TSH
If thyrotoxicosis is due to a rarer pituitary cause, then there will be an increased production of pituitary TSH, causing the increased production of thyroid hormones. The pattern of TFT results will be as follows:9
- Increased T3 and T4
- Increased TSH
Autoantibodies
The presence of thyroid autoantibodies suggests there is an underlying autoimmune disease causing thyrotoxicosis. The presence of anti-TSHR antibodies is highly suggestive of Grave’s disease.10
Other autoantibodies suggestive of autoimmune thyroid disease include anti-thyroid-peroxidase (anti-TPO) antibodies and anti-thyroglobulin (anti-Tg) antibodies. These are non-specific and can be present in autoimmune causes of both hyperthyroidism (Grave’s disease) and hypothyroidism (Hashimoto’s disease).4
Imaging
Doppler ultrasound may be used to image the thyroid gland, to confirm the presence of goitre and identify nodules.11
Management
Beta-blockers
Beta-blockers (commonly propranolol) are indicated in the management of thyrotoxicosis to provide symptomatic relief from the typical adrenergic symptoms (palpitations, tachycardia, tremor).4,12
Block and replace
The block and replace management regime is commonly used for the management of hyperthyroidism in adults and is the first-line management option for hyperthyroidism in children.
Block and replace involves blocking the excess thyroid hormone production from the thyroid gland and replacing this with the correct concentration of exogenous thyroid hormones.
Carbimazole is used to block the thyroid hormone production and levothyroxine is used to replace the thyroid hormones (these medications are administered simultaneously). Patients require ongoing monitoring of their TFTs and their FBC (due to the risk of agranulocytosis associated with carbimazole).
In most patients with Grave’s disease, a block and replace management regime can induce remission.12
Radioiodine
Radioiodine is a definitive management option for Grave’s disease and toxic multinodular goitre.
If radioiodine is deemed unsuitable for patients with Grave’s disease (e.g. due to pregnancy or malignancy), block and replace therapy is typically used as a second-line management option.
If radioiodine is deemed unsuitable for patients with a toxic multinodular goitre, thyroid surgery is used as a second-line management option.
Radioiodine can also be used second-line in patients with hyperthyroidism in whom block and replace has been unsuccessful.2
With radioactive iodine, measures must be taken following treatment to prevent any harm coming to those in close contact with the patient. Patients must be advised against prolonged contact with children (including their own) and pregnant women.
Female patients must also be advised against becoming pregnant within the next six months following treatment and male patients must be advised against fathering children for the next four months following treatment.12
Surgery
Thyroidectomy (complete removal of the thyroid gland) may be considered for the management of hyperthyroidism.
Thyroidectomy is indicated in cases of thyroid malignancy, or where a thyroid goitre is causing compression of surrounding structures. Thyroidectomy may also be considered if other treatment options are unsuitable or unsuccessful.12
Removal of the thyroid gland will result in a lack of circulating thyroid hormones in these patients. Therefore, they will need to be started on long term thyroid hormone replacement, most commonly levothyroxine.4
Complications
Thyroid storm
A serious complication of thyrotoxicosis is the onset of a thyroid storm, which involves excessive adrenergic activity secondary to thyrotoxicosis.
Clinical features of a thyroid storm may include:13
- Palpitations
- Tachycardia (often greater than 140 beats per minute)
- Tremor
- Nausea and vomiting
- Abdominal pain
- Reduced level of consciousness
- Confusion/agitation
- Seizures
Thyroid storm is associated with high mortality.
Cardiac complications
Cardiac complications of thyrotoxicosis include:4
- Atrial fibrillation: can be seen in up to 10-25% of patients. These patients require management of their hyperthyroidism as previously described as well as rate control (e.g. beta-blocker) and anticoagulation (e.g. Warfarin or Apixaban).
- Heart failure: this is more prevalent in elderly patients with hyperthyroidism, secondary to cardiomyopathy occurring in long-term thyrotoxicosis.
- Angina: should be considered in hyperthyroid patients presenting with exertional chest pain.
Grave’s ophthalmopathy
It is important to inform patients of the complications of Grave’s ophthalmopathy. In some instances, this can also cause deterioration of visual acuity if the optic nerve is affected.14
Key points
- Thyrotoxicosis is the clinical manifestation of excess thyroid hormone action at the tissue level due to inappropriately high circulating thyroid hormone concentrations.
- Hyperthyroidism is a subset of thyrotoxicosis, referring specifically to excess thyroid hormone synthesis and secretion by the thyroid gland.
- Thyroid hormone production is regulated via the hypothalamic-pituitary-thyroid (HPT) axis.
- Common causes of thyrotoxicosis include Grave’s disease and toxic multinodular goitre.
- Clinical features of thyrotoxicosis occur due to an abnormally increased basal metabolic rate.
- Clinical features specific to Grave’s disease include thyroid eye disease, thyroid acropachy and pretibial myxoedema.
- Thyroid function tests and autoantibody status can allow the underlying cause of thyrotoxicosis to be established.
- Beta-blockers (e.g. propranolol) are used to manage the adrenergic clinical features of thyrotoxicosis (e.g. tachycardia, tremor).
- First-line pharmacological management of thyrotoxicosis involves the use of a block (carbimazole) and replace (levothyroxine) regime.
- Other management options include radioiodine and thyroidectomy.
Thyroid function tests (TFTs) are a commonly requested investigation in clinical practice. This guide aims to provide you with the knowledge needed to interpret TFTs and consider underlying pathology.
Thyroid hormonal axis
The ability to interpret thyroid function tests (TFTs) first requires an understanding of the thyroid hormonal axis. Each of the thyroid function tests assesses a different aspect of this axis and understanding the relationship between the results is key to being able to reach a diagnosis.
Thyroid hormonal axis overview
1. The paraventricular nuclei in the hypothalamus release thyroid-releasing hormone (TRH).
2. This causes thyrotrope cells in the anterior pituitary to release thyroid-stimulating hormone (TSH).
3. The thyroid responds to the TSH by releasing T4 and T3.
4. T4 inhibits the pituitary and hypothalamus in a negative feedback loop. This is the ‘brake system’ which aims to maintain a state of homeostasis.
Thyroid function tests (TFTs)
The term ‘thyroid function tests‘ refers to the following investigations:
- TSH (0.4 – 4 mU/L)
- Free T4 (9 – 25 pmol/L)
- Free T3 (3.5 – 7.8 nmol/L)
There are separate reference ranges for children and pregnant women.
Reference ranges for TFTs often vary between labs, so always refer to your local guidelines.
Important points
Whilst free T3 (fT3) is measured, it is less relevant than free T4 (ft4). This is because the thyroid releases T4 and T3 at a ratio of about 20:1 respectively, with T3 mainly being produced by peripheral conversion of T4. As a result, T4 is a much better marker of thyroid function.
Free T4 (fT4) is roughly 1% of the total T4, with the rest being bound to thyroid-binding globulin.
T4 has a half-life of about one week, therefore, to monitor the impact of an intervention (e.g. increasing a patient’s levothyroxine dose) you need to wait several weeks before repeating TFTs.
Hypothyroidism
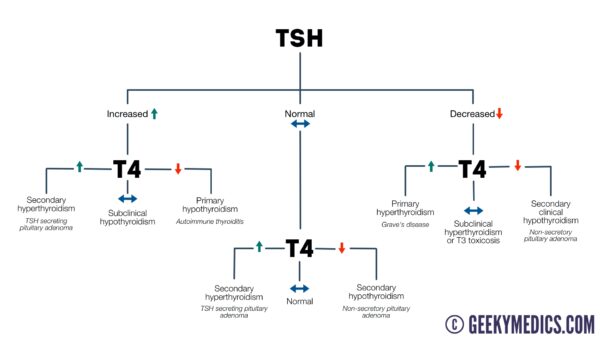
Primary hypothyroidism
Primary hypothyroidism involves reduced secretion of thyroid hormone from the thyroid gland itself.
Pathology which decreases the thyroid’s ability to release T4 and T3 or respond to TSH can, therefore, cause primary hypothyroidism.
Primary hypothyroidism is the most common cause of hypothyroidism, accounting for 99% of all cases.
Pathophysiology
1. Less T4 and T3 are produced due to the thyroid’s reduced capacity to produce hormone or respond to TSH.
2. As a result, there is reduced negative feedback on the pituitary and hypothalamus.
3. The reduction in negative feedback results in increased production of TRH (which we don’t typically measure) and TSH.
4. The end result is low T4 and T3 and a raised TSH.
Diagnosis
The typical findings that would indicate a diagnosis of primary hypothyroidism are as follows:
- Raised TSH: due to the absence of negative feedback.
- Low T4: due to the thyroid’s inability to produce enough T4.
A normal T4 in the context of a raised TSH may suggest subclinical hypothyroidism (most commonly caused by underlying autoimmune disease).
Aetiology
Causes of primary hypothyroidism include:
- Autoimmune thyroiditis (50%)
- Iodine deficiency or excess
- Thyroidectomy
- Therapy with radioactive iodine – a treatment for hyperthyroidism
- External radiotherapy
- Drugs
- Thyroid agenesis or dysgenesis
Secondary hypothyroidism
Secondary hypothyroidism involves a reduction in the hormones that stimulate the thyroid to produce thyroxine.
Pathology which affects the pituitary and hypothalamic glands can result in decreased production of TRH and TSH, causing secondary hypothyroidism.
Secondary hypothyroidism is a rare cause of hypothyroidism, accounting for 1% of all cases.
Pathophysiology
1. Decreased production or secretion of TRH and TSH results in decreased stimulation of the thyroid gland.
2. The thyroid gland, therefore, produces less T3 and T4.
3. The low T3 and T4 would normally stimulate the pituitary and hypothalamic glands to increase TRH and TSH production, however, they are unable to increase production.
4. The end result is low T4 and T3 and a normal/low TSH.
Diagnosis
The typical findings that would indicate a diagnosis of secondary hypothyroidism are as follows:
- Normal/low TSH: due to a lack of production.
- Low T4: due to the absence of any positive feedback from TSH.
Aetiology
Causes of secondary hypothyroidism can be either pituitary or hypothalamic in origin.
Pituitary causes:
- Pituitary adenoma: the most common cause.
- Pituitary surgery or radiotherapy which damages the pituitary tissue.
Hypothalamic causes:
- Hypothalamic or suprasellar tumour.
- Surgery or radiotherapy which damages the hypothalamic tissue.
TRH is not measured as part of thyroid function tests, as it is only released locally between the hypothalamus and pituitary (so it is not present in representative quantities within the peripheral circulation). Both structures are commonly grouped together into ‘secondary’ because an issue with TRH gives the same blood test results as an issue with TSH, however, some literature may refer to the hypothalamus as a ‘tertiary’ cause.
Hyperthyroidism
Primary hyperthyroidism
Primary hyperthyroidism involves an excessive production of T3 and T4 by the thyroid gland as a result of pathology within the thyroid gland itself.
Pathophysiology
1. The thyroid produces excessive amounts of T4 and T3.
2. The excessive T4 and T3 cause negative feedback on the pituitary and hypothalamus, resulting in decreased production of TRH and TSH.
3. The end result is a raised T3 and T4 and a low TSH.
Diagnosis
The typical findings that would indicate a diagnosis of primary hyperthyroidism are as follows:
- Raised T3/T4: due to excessive production.
- Low TSH: due to negative feedback on the pituitary/hypothalamus.
Aetiology
Causes of primary hyperthyroidism include:
- Graves’ disease (75% of all cases)
- Toxic multinodular goitre
- Toxic adenoma
- Iodine-induced (rare)
- Trophoblastic tumour (very rare)
Secondary hyperthyroidism
Secondary hyperthyroidism involves stimulation of the thyroid gland by excessive thyroid-stimulating hormone (TSH).
Pathophysiology
1. TSH production is increased by either the pituitary/hypothalamus or another source (known as ectopic production).
2. The excess TSH causes overstimulation of the thyroid gland, resulting in high levels of T3 and T4 production.
3. Normally a raised T3 and T4 level would cause negative feedback, decreasing TSH production, however, in this instance, the TSH production is not responsive to any negative feedback, resulting in continued excess production.
Diagnosis
The typical findings that would indicate a diagnosis of secondary hyperthyroidism are as follows:
- Raised T3/T4: due to excess production that is driven by a raised TSH level.
- Raised TSH: due to excess production.
Aetiology
Causes of secondary hyperthyroidism include:
- TSH-secreting tumour
- Chorionic-gonadotropin secreting tumours (hCG secreting)
- Thyroid hormone resistance (usually euthyroid): TSH is resistant to T3/T4 negative feedback.
Summary
Below is a flowchart that summarises the key points discussed in this article.
Reviewer
Professor Levy
Consultant Endocrinologist
References
- The endocrine system at a glance, 3rd edition; B.Greenstein D.Wood; Wiley-Blackwell; ISBN 978-1-4443-3215-5; 2011
- Comprehensive Clinical Endocrinology 3rd ed., G.Besser, M. Thorner; 2002; 0-7234-3185-X; Elsevier science limited
- Willims Textbook of Endocrinology 10th ed.; Larsen, Kronenberg, Melmed, Polonsky; 0-7216-9184-6; 2003
- De Leo, Lee, Braverman; Hyperthyroidism; Lancet. 2016 Aug 27; 388(10047): 906–918; available from: [LINK].
- Kostoglou-Athanassiou, Ntalles; Hypothyroidism – new aspects of an old disease; Hippokratia, 2010 Apr-Jun; 14(2): 82-87
- Dr Lousie Newson;Thyroid Function Tests. Patient.info. Published in 2015. Available from: [LINK].
Reviewer
Dr Peter King
Reader in Molecular Endocrinology
Editor
Dr Chris Jefferies
References
Text references
- GeekyMedics.com
- Parveen K, Michael C. Kumar and Clark’s Clinical Medicine. 9 ed. London: Elsevier; 2017.
- Moore K, Dalley A, Agur A. Clinically Oriented Anatomy. 7 ed: Lippincott Williams and Wilkins 2013. 1168 p.
- Rhoades R, Bell D. Medical Physiology. 4 ed. Philadelphia: Lippincott Williams & Wilkins 2013. p. 621-8.
- Wilkinson I, Raine T, Wiles K, Goodhart A, Hall C, O’Neil H. Oxford Handbook of Clinical Medicine. 10 ed. Oxford: Oxford University Press; 2017.
- Kim HY, Mohan S. Role and Mechanisms of Actions of Thyroid Hormone on the Skeletal Development. Bone Res. 2013;1(2):146-61.
- BMJ Best Practice. Toxic multinodular goitre. 2019. Available from: [LINK].
- NICE CKS. Hyperthyroidism. 2020. Available from: [LINK].
- Jadidi J, Sigari M, Efendizade A, Grigorian A, Lehto SA, Kolla S. Thyroid acropachy: A rare skeletal manifestation of autoimmune thyroid disease. Radiol Case Rep. 2019;14(8):917-9.
- Starr O, Tidy C. Thyroid Function Tests. 2020. Available from: [LINK].
- BMJ Best Practice. Graves’ disease. 2020. Available from: [LINK].
- NICE. Hyperthyroidism: Diagnosis. 2020. Available from: [LINK].
- NICE. Hyperthyroidism: Management. 2020. Available from: [LINK].
- Newson L, Bonsall A. Hyperthyroid Crisis (Thyrotoxic storm). 2015. Available from: [LINK].
- Foundation BT. Thyroid Eye Disease. 2019. Available from: [LINK].
Image references
- Figure 1. Geeky Medics. The hypothalamic-pituitary-thyroid (HPT) axis.
- Figure 2. Jonathan Trobe, M.D. Thyroid eye disease. License: [CC-BY]. Available from: [LINK]
- Figure 3. Herbert L. Fred, MD and Hendrik A. van Dijk. Thyroid acropachy. License: [CC-BY]. Available from: [LINK]
- Figure 4. Herbert L. Fred, MD and Hendrik A. van Dijk. Pretibial myxoedema. License: [CC-BY]. Available from: [LINK]